- Stimulated emission-assisted amplification of fluorescence
-
Fluorescence is a ubiquitous phenomenon which stems from the spontaneous relaxation of an excited state by an emitter with a dipole-allowed transition. Among its extremely varied implications, fluorescence plays a fundamental role in chemical and biochemical diagnostics. Decades ago it has gain prominence as the workhorse of assisted imaging in the life sciences for its ability to highlight cellular subconstituents or functions, thus enabling not only the visualization of cell parts whose contrast would be too poor in image recognition, but also the conversion of biochemical processes into optical signals. In a way, fluorescence in biochemistry has played the role of transducer which transforms the nature of the signal, like current photodiodes, thermoconductor, etc., convert the measurement of the physical quantity for which they are designed into an electrical signal.
In spite of the extreme diffusion of fluorescence, it is not uncommon for an interesting biochemical signal to remain undetected or very poorly detected. This is due to various sources of background (from technical noise to autofluorescence of tissues) which partially or entirely mask the signal of interest. Thus, it is desirable to find selective amplification mechanisms capable of enhancing the optical signal of a particular class of emitters to make its signal emerge from noise. While biochemical amplifiers are being developed for this purpose, they remain very expensive, limited in number and scope, due not only to costs but also to the physical space that the amplifying molecules occupy -- a constraint which may be incompatible with the (sub-)cellular function of interest.
In a collaboration with the IPMC (F. Brau), we have been developing an amplification mechanism based on stimulated emission: the fundamental process which leads to lasing. In order to avoid the complications of external reflectors (mirrors), our goal has been to obtain the amplification through random scatterers added to the physiological solution in which cells live. While the idea is in itself known -- as random lasers have been realized, even with biological specimen -- its implementation under strictly biocompatible conditions is far from trivial, since biotoxicity of scatterers, dye and pump light have to be avoided and issues related to scatterer clustering have to be tackled, in an environment (rich in ions) which favours the clumping of the nanoparticles and their subsequent "neutralization" as effective scatterers.
This line of research has been advanced with the help of one doctoral student (S. Bonnefond) and three Master students (F. Audot, I. Grosjean and A. Raynaud), all graduated, and has led to a successful realization of amplification.
Further developments, now underway, include the consideration of aerogels as supports for the scatterers (collaboration with T. Budtova -- CEMEF, Mines ParisTech; Master Project of Y. Yiang, 2020-21) and advance mathematical modelling (Atlantis collaboration of INRIA Méditerranée).
- Non-invasive interferometric measurement of acoustomechanical cell deformation
-
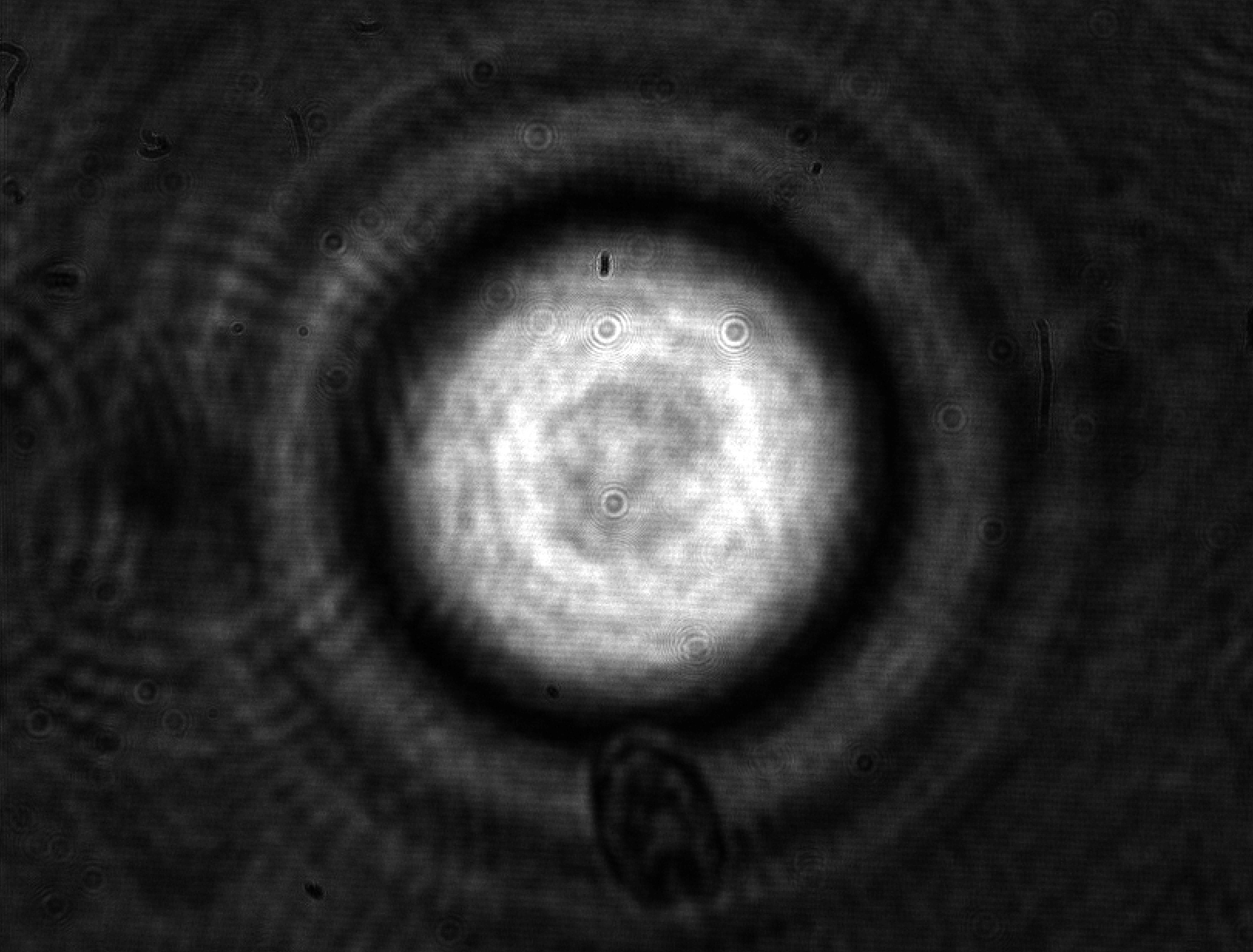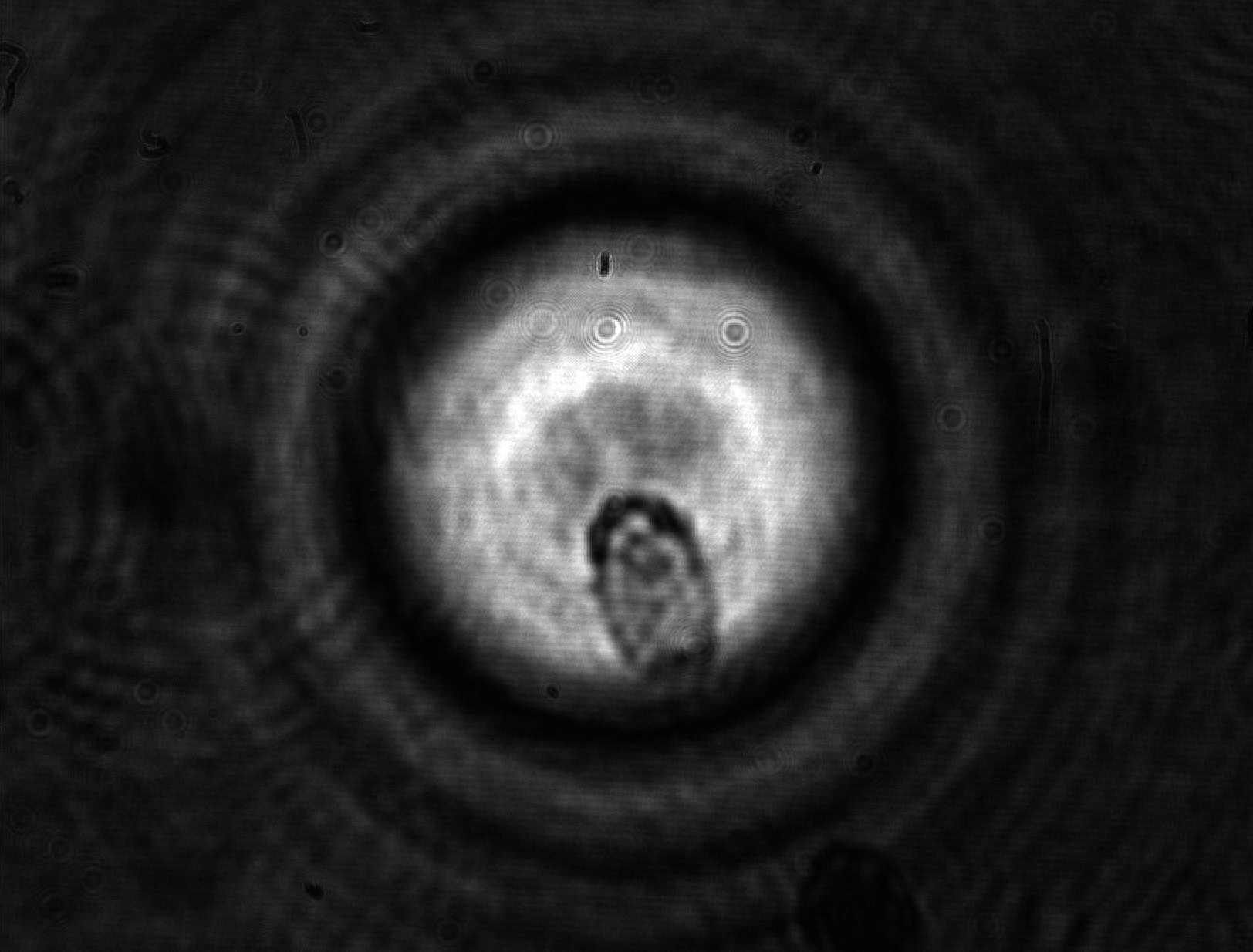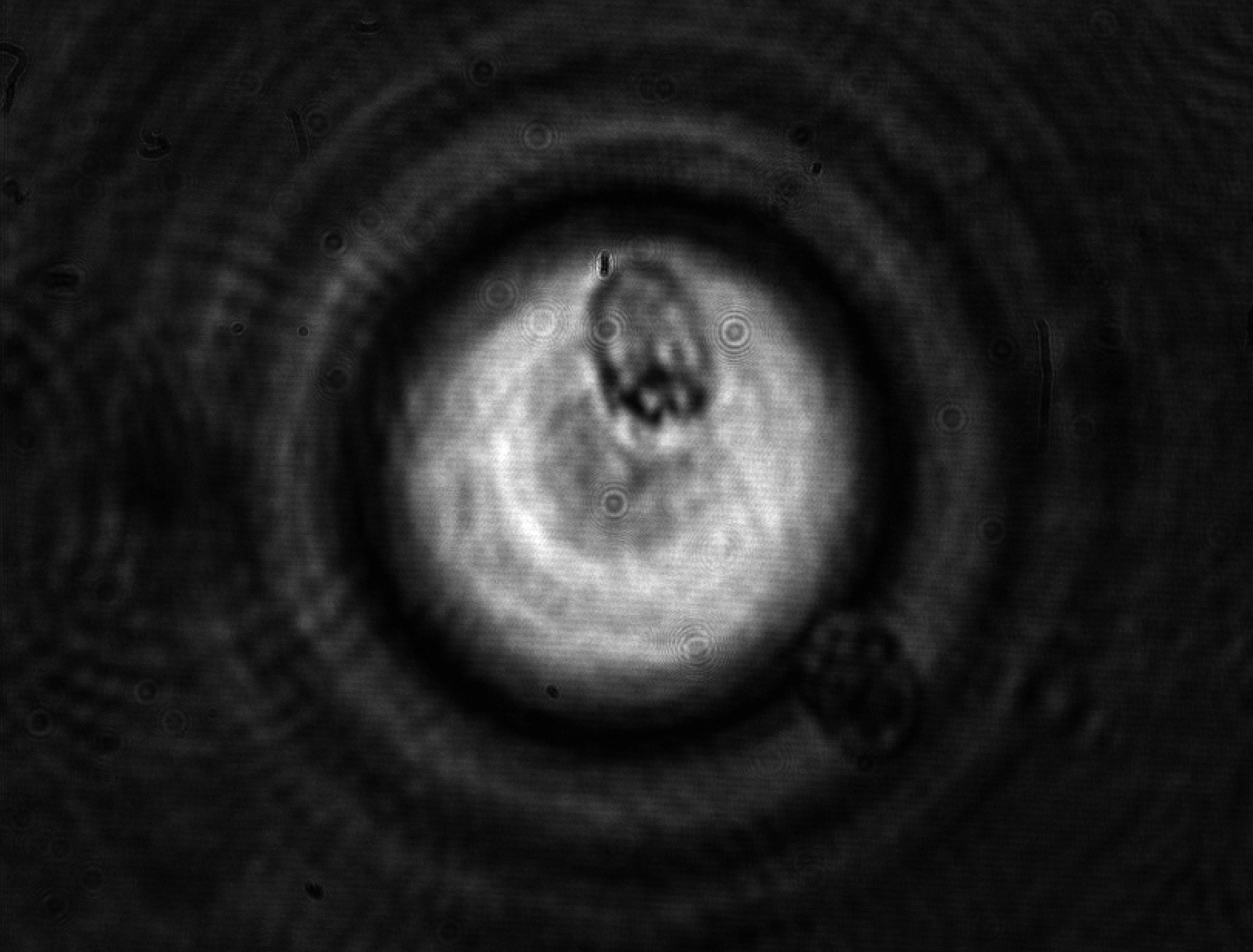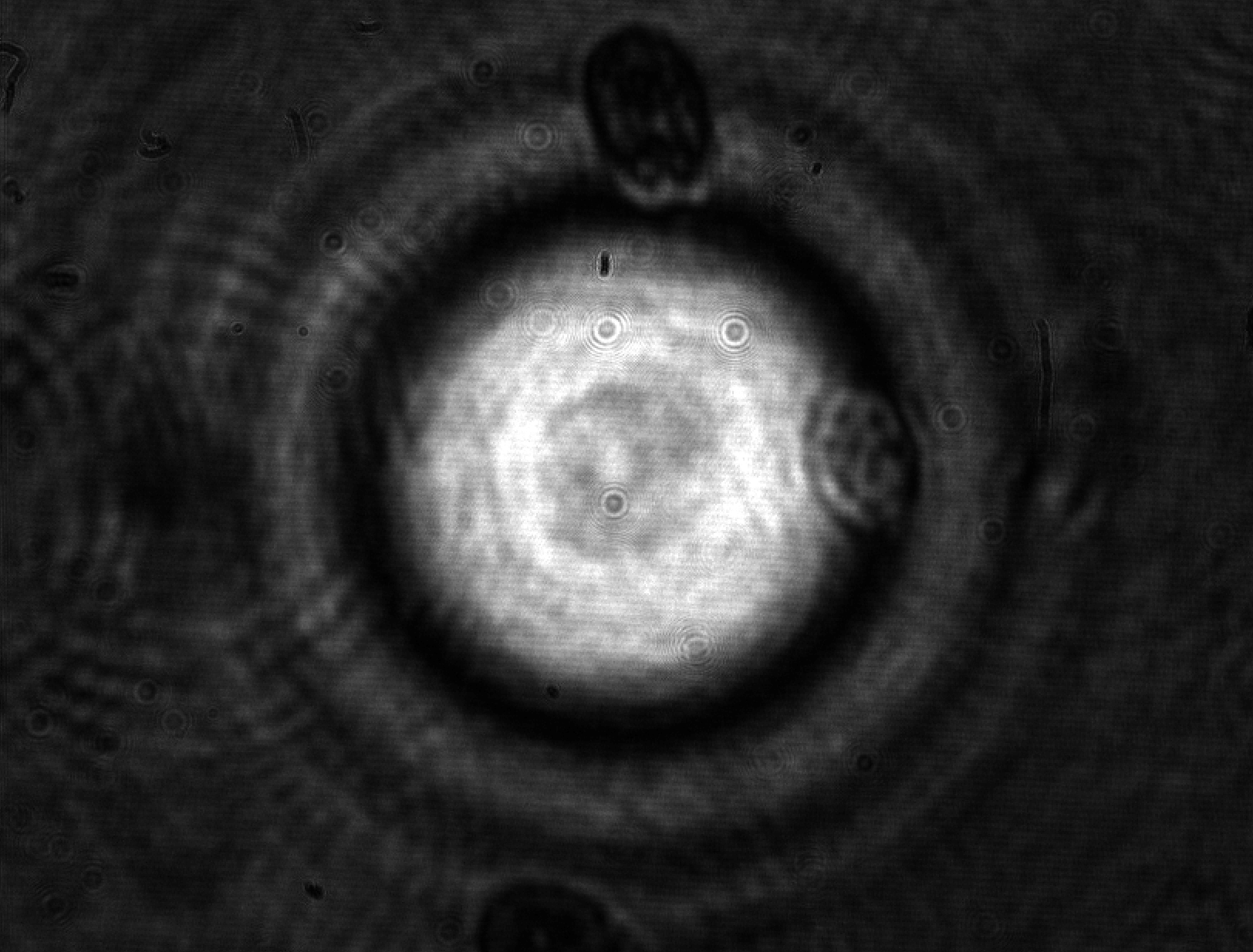
Cell mechanical properties have been emerging as a factor probably as important as the biochemical processes which govern its life and evolution. Cells diversify and develop on the basis of the mechanical properties of the environment and change their viscoelasticity depending on their health state. For instance, malaria is now known to stiffen red blood cells, obstructing their transit through narrow blood vessels and causing, as a consequence, organ functional problems.
Cell mechanical properties are struggling to gain sufficient recognition in the life sciences and, even more, in medicine due in part to the lack of adequate instrumentation capable of providing sufficiently reliable measurements. Standard mechanical tests, such as those performed with Atomic Force Microscopy (AFM) are liable to provide widely differing results even on a same cell depending on the tip used for the contact and the amount of applied force. Models accounting for the shape and contact surface cannot actually compensate for the discrepancies. This shortcoming is not specific to the AFM, but is common to virtually all mechanical techniques based on contact: the shape and kind of constraint bias the outcome in a substantial way. A second shortcoming of most mechanical measurements comes from their low throughput, which can be measured in units or tens or units per hour -- aside from a couple of exceptions. This limitation, combined with the high variability in intrinsic properties typical of all living organisms, is responsible for the limited recognition of biomechanics and for the lost opportunities for cell studies and diagnostics.
In a collaboration with the University of Glasgow (M. Vassalli) and the University of Southampton (P. Glynne-Jones), we have been developing an instrument based on interferometric optomechanical measurements where the constraint is applied by a high-frequency acoustic field (thus compressing the whole cell) to cells which flow in a microfluidic channel. This way, both the mechanical pressure and the measurement are globally applied to the whole cell and are contactless, avoiding the pitfalls of the classical techniques. Currently, deformations applied to algae and yeast cells are well characterized by the instrument (fruit of the Ph.D. thesis work of Julián Mejía) with good statistical significance.
Future instrumental developments are expected to enable sufficiently fast rates for the acquisition of statistically significant samples in reasonable measurement times.
lippi